Click chemistry
Click chemistry is a versatile technique that can be used for the synthesis of a variety of conjugates. Virtually it can be applied to any biomolecules.
„It just says click – and the molecules are coupled together“ (1)
With respect to oligonucleotide labeling with small molecules, such as fluorescent dyes, biotin, and alike, click chemistry opens doors to a whole "new world" of exciting and so far unimaginable or de facto commercially rather unrealizable molecule specifications.
The concept of "Click Chemistry" has been coined by Nobel Laureate K. Barry Sharpless around 2000 to identify a reliable and straightforward form of chemistry in which reactions take place fast with the absence of undesired by-products.
A little later, and independently of each other, Morten Meldal and Barry Sharpless described the [3+2] cycloaddition between alkynes and azides (2).
Although around 1960 Rolf Huisgen first reported this typical "click" reaction, the 1,3-dipolar cycloaddition (3, 4), it was only Sharpless and Meldal who subsequently described it as a copper-catalyzed process (Figure 1, 5, 6). The two components - azido and alkyne groups - are nearly never encountered in natural biomolecules. Hence, conjugation reactions arrive at a remarkable degree of selectivity and specificity (7).
Figure 1. The click reaction.
Applying this concept on, and further developing it in the field of Nucleic Acid modification, Thomas Carell's work has been turning click chemistry into a very hot topic for oligonucleotide labeling (Figure 2, 8).
Figure 2. Oligonucleotide labeling via click chemistry. The further development of the concept to modification of nucleic acids by Thomas Carell's working group not only concerns the labelling of individual oligonucleotides with regard to the introduction of multiple modifications, but also the attachment of terminal labels to oligos that exceed a critical length.
Respective citations have been, are, and will be growing rapidly.
Prof. Dr. Thomas Carell is one of the founders of baseclick GmbH, headquartered in Tutzing/Munich. Turning his expertise into a successful business idea, baseclick GmbH was granted the basic patent on copper-catalyzed click reactions on nucleic acids by the European Patent Office in 2010. metabion has been offered a license for the respective labeling processes including an extended collaboration, which we happily assumed.
Always striving to be at the forefront of technical improvements in order to serve our customers best, metabion has secured a worldwide license for employing this interesting chemistry on producing modified oligonucleotides from baseclick GmbH.
Additionally and by using the same technology platform, click chemistry also allows for multiple labeling of polymerized DNA strands via incorporation of Alkyne-dNTPs (triphosphates) followed by post-synthetic click reactions at a so far unmatched efficiency level (Figure 3, 8, 9).
Figure 3. Multiple labelling of probes. Through click chemistry, unprecedented levels of labelling can be achieved: the incorporation of alkyne-labelled dNTPs by nick translation or PCR allows for customised ready-to-use/fully labelled oligonucleotides.
For living systems, unfortunately, the copper catalyst itself is toxic, but an innovative solution to this problem emerged in 2004, when scientist Carolyn Bertozzi found and published an alternative to copper ions to carry out the azide-alkaline reaction in cells (10). It all started when, in the early 1990s, she was attempting to map a glycan that attracted immune cells to lymph nodes. During a seminar, Carolyn Bertozzi listened to a German scientist explaining how he had managed to get cells to produce an unnatural variant of sialic acid, and thought of applying a similar method to get cells to incorporate a modified sialic acid with a type of chemical manipulation, e.g. a fluorescent molecule, into different glycans so that one could map hidden glycans within the cell (Figure 4, 10, 11).
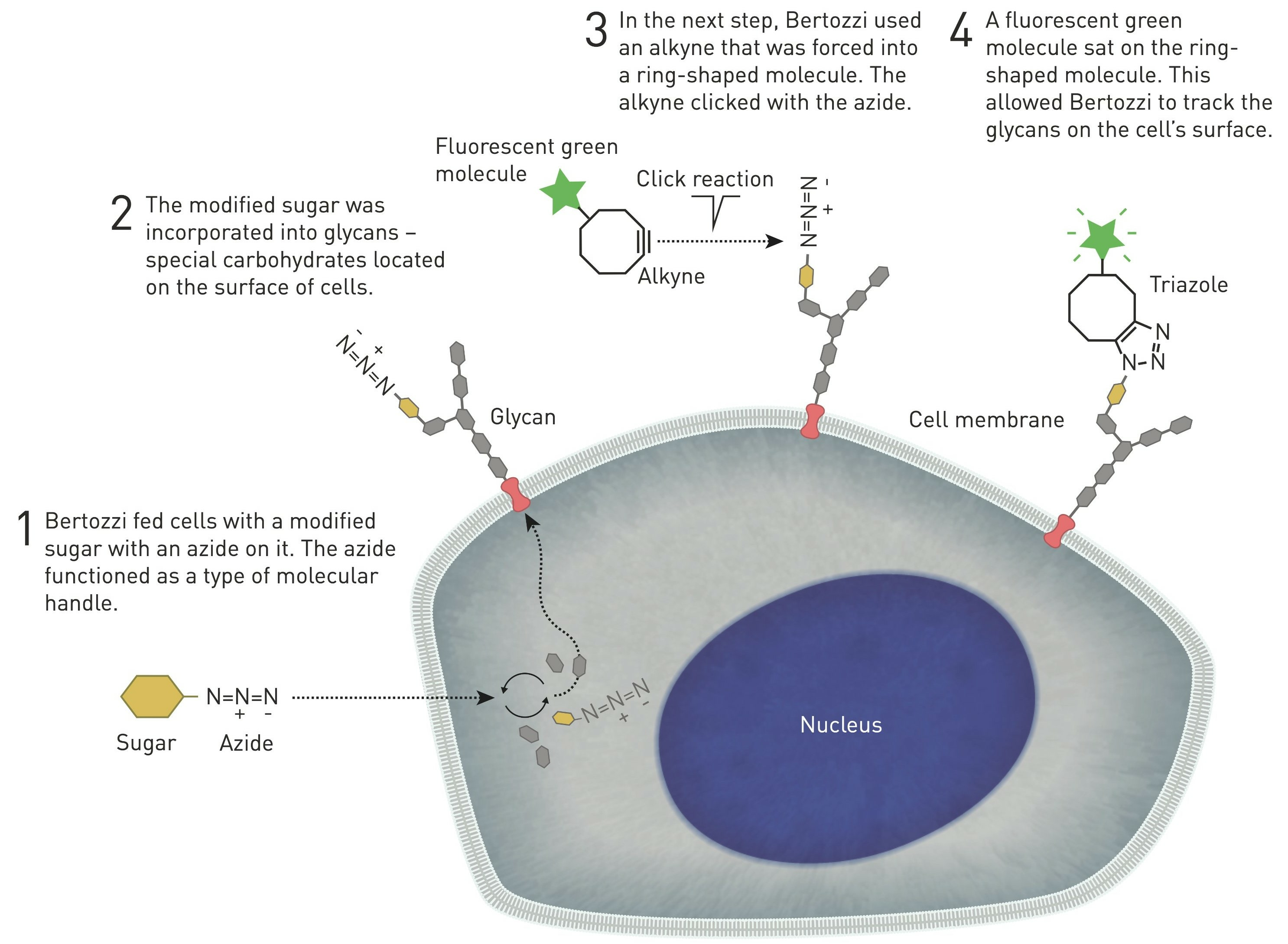
Figure 4. Graphical representation of bio-orthogonal reactions in the cell (© Johan Jarnestad, 1).
Bertozzi removed the copper catalyst from the reaction by inserting the alkyne in a ring structure, which advances the reaction by exploiting the ring strain produced by the cyclically constrained molecules. In this way, such reactions that occur "in parallel" with the chemical environment of the cell, bio-orthogonal reactions, can proceed in cells without disrupting their normal chemistry (Figure 4, 10, 11). This strain-mediated click reaction allowed the glycans to be followed within the cell (Figure 5, 12).
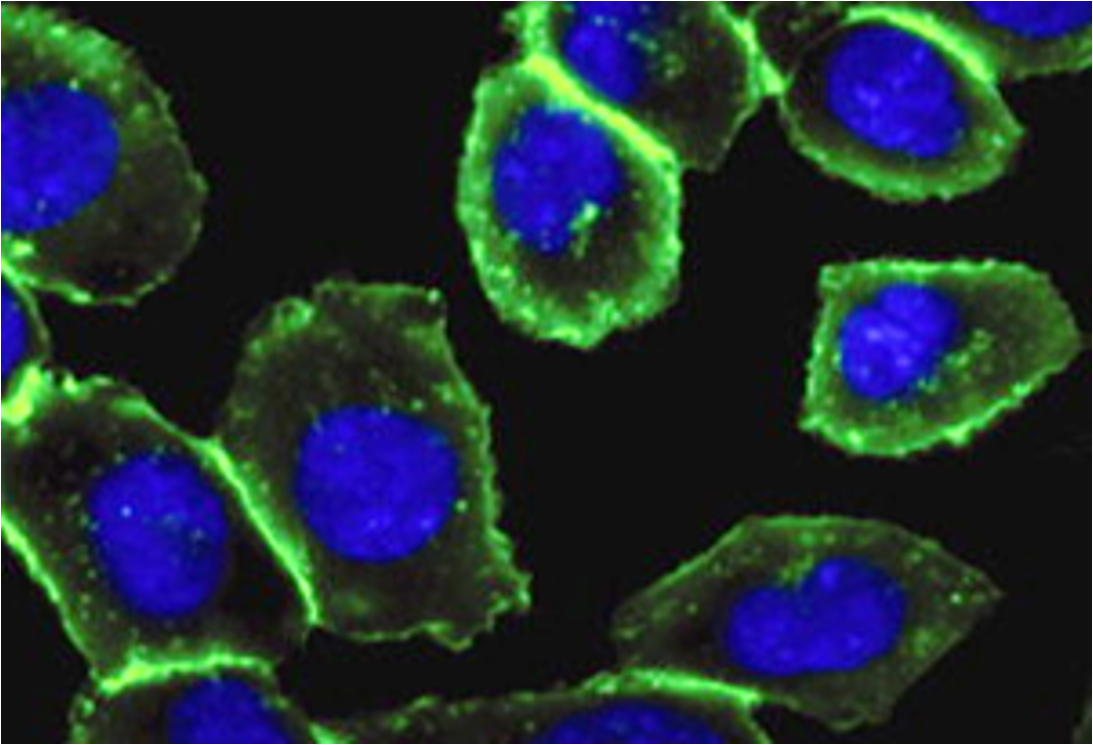
Figure 5. Imaging of glycans in the cell by labelling with Cu-free click chemistry: labelled cell-surface glycans (green glow) and cell nucleus (blue, modified from 12).
Hence, bio-orthogonal chemistry has marked a new milestone in click
chemistry that leads to a new dimension in which click reactions
function within living organisms, enabling researchers to visualize and
follow molecules in living cells in their natural state without
influencing them.
As a crowning achievement, scientists Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless were awarded the 2022 Nobel Prize in Chemistry for their development of click chemistry and bio-orthogonal chemistry (Figure 6, 1).
Figure 6. The Nobel Prize in Chemistry 2022 (© Johan
Jarnestad, 1).
Consequently, it is intuitive that the number of areas in which click
chemistry and bio-orthogonal reactions have been employed has
significantly risen (13). Researchers have enhanced the targeting of
anti-cancer drugs, without perturbing healthy cells, which are now
undergoing clinical trials (14). Bertozzi herself, through her studies on
glycans, is
scientific advisor of a company which has developed a pharmaceutical drug, currently in phase II clinical trials on advanced
cancer patients (15), capable of breaking down the glycans, present on the
surface of cancer cells, which in some cases are able to protect these
cells from attack by the immune system (16). Anticorps have also been
designed to collimate tumour cells using the click response and then be
attacked by a second molecule. Likewise, large quantities of polymers
for building materials have been produced sustainably and rapidly.
"Click" in summary...
- New, quick and easy reaction in water;
- Compatible with many functions, non-solvent sensitive;
- Very sensitive products can be connected;
- No by-products are generated;
- It possesses several applications in biochemistry, polymers, drug discovery…. and the most important: labelling of biomolecules with dyes;
- Bio-orthogonal Click for copper-free applications in live cells which makes processes in the cell visible.
Click chemistry and its potential for new drugs and new treatments
are still a long way off, but the game is on.
So, what are you waiting
for? Check out our Portfolio!
What we offer
“ready-to-use/fully labeled” custom oligonucleotides
Having successfully introduced this additional labeling procedure for synthetic Nucleic Acids and other biomolecules into our custom synthesis operations, we are now able to offer significantly increased flexibility in terms of:
- multiple labeling of oligonucleotides (2 or more modifications);
- producing modified oligonucleotides exceeding a critical sequence length.
While in the past state-of-the-art chemistry and technologies either did not allow for the above options at all or if anything, not in a commercially reasonable and affordable way, we are happy to provide new solutions for your innovative ideas.
“ready-to-click-it-yourself” custom oligonucleotides
Moreover, we can provide you with alkyne-modified oligonucleotides to
be labelled at your end by “clicking” the desired “Azide-modification”.
Click here for our Azide-portfolio.
“modimerization” solutions
In line with above´s “click it yourself” approach, we can also supply you with Alkyne Nucleotides (triphosphates) to be incorporated into DNA during polymerization reactions (PCR, reverse transcription, nick-translation, etc.). This prepares the produced DNA strands for post-synthetic “click” conjugations to azide-activated labels – a very elegant and efficient way of “modimerizing” DNA strands.
Applications
Extended means of (multiple) labeling of biomolecules such as Nucleic Acids (DNA/RNA), peptides, proteins, and antibodies will have a vigorous effect on the following areas (making no claim to be exhaustive):
- Fluorescence In Situ Hybridization (FISH)
- qPCR-Assays
- DNA/RNA Microarrays
- Bioconjugation to Nanoparticles
- Fluorescence Microscopy
- Electrophoretic Mobility Shift Assay (EMSA)
- Fluorescence Activated Cell Sorting (FACS)
- Flow Cytometry (FCM), Cell Tracking and Cell-based Assays
- Fluorescence Correlation Spectroscopy (FCS)
- ... We at metabion use it daily to produce fluorescent DNA for dyes that do not survive/are not available for synthesis.
References
- The Royal Swedish Academy of Sciences. The Nobel Prize in Chemistry 2022. Press release. NobelPrize.org. Nobel Prize Outreach AB 2023.
- Kolb HC., Finn MG., Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001 Jun 1;40(11):2004-2021.
- Huisgen R. Centenary Lecture—1,3-Dipolar Cycloadditions. Proc. Chem. Soc. Lond. 1961, 357–396.
- Breugst M., Reissig HU. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew Chem Int Ed Engl. 2020 Jul 20;59(30):12293-12307.
- Tornøe CW., Christensen C., Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002 May 3;67(9):3057-64.
- Rostovtsev VV., Green LG., Fokin VV., Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective "ligation" of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002 Jul 15;41(14):2596-9.
- Worrell BT., Malik JA., Fokin VV. Direct evidence of a dinuclear copper intermediate in Cu(I)-catalyzed azide-alkyne cycloadditions. Science. 2013 Apr 26;340(6131):457-60.
- Gierlich J., Burley GA., Gramlich PM., Hammond DM., Carell T. Click chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org Lett. 2006 Aug 17;8(17):3639-42.
- Fantoni NZ., El-Sagheer AH., Brown T. A Hitchhiker's Guide to Click-Chemistry with Nucleic Acids. Chem Rev. 2021 Jun 23;121(12):7122-7154.
- Agard NJ., Prescher JA., Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004 Nov 24;126(46):15046-7.
- Dube DH., Bertozzi CR. Metabolic oligosaccharide engineering as a tool for glycobiology. Curr Opin Chem Biol. 2003 Oct;7(5):616-25.
- Baskin JM., Prescher JA., Laughlin ST., Agard NJ., Chang PV., Miller IA., Lo A., Codelli JA., Bertozzi CR. Copper-free click chemistry for dynamic in vivo imaging. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):16793-7.
- Scinto SL., Bilodeau DA., Hincapie R., Lee W., Nguyen SS., Xu M., Am Ende CW., Finn MG., Lang K., Lin Q., Pezacki JP., Prescher JA., Robillard MS., Fox JM. Bioorthogonal chemistry. Nat Rev Methods Primers. 2021;1:30.
- Datz S., Argyo C., Gattner M., Weiss V., Brunner K., Bretzler J., von Schirnding C., Torrano AA., Spada F., Vrabel M., Engelke H., Bräuchle C., Carell T., Bein T. Genetically designed biomolecular capping system for mesoporous silica nanoparticles enables receptor-mediated cell uptake and controlled drug release. Nanoscale. 2016 Apr 21;8(15):8101-10.
- Williams J. Shasqi, Inc. A Multicenter Phase 1/2a, Open-Label Study of SQ3370 in Patients With Advanced Solid Tumors. ClinicalTrials.gov Identifier: NCT04106492. Last Update: February 9, 2023. Accessed August 8, 2023.
- Srinivasan S., Yee NA., Zakharian M., Alečković M., Mahmoodi A., Nguyen TH., Mejía Oneto JM. SQ3370, the first clinical click chemistry-activated cancer therapeutic, shows safety in humans and translatability across species. bioRxiv [Preprint]. 2023 Mar 29:2023.03.28.534654.

