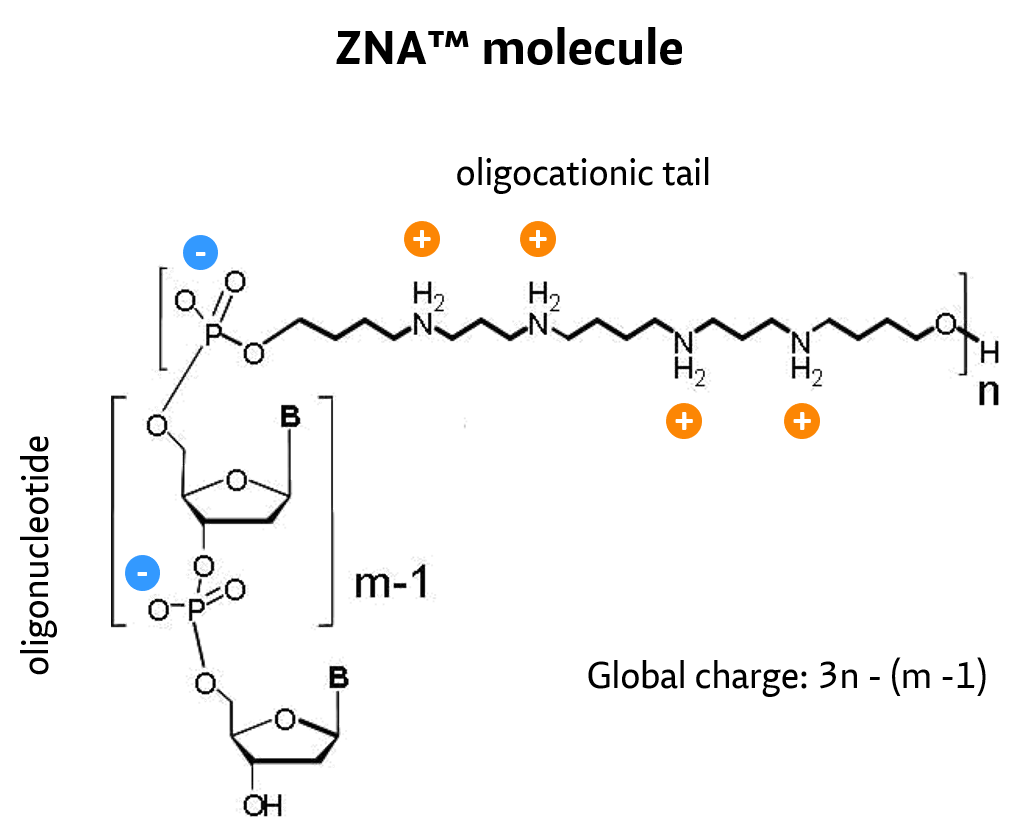
What is the concept of ZNA®?
Oligonucleotides are at the heart of some of the most powerful molecular biology techniques like PCR, DNA chips and in situ hybridization. Moreover, lots of hope has been pinned for oligonucleotides as a generic, yet very selective, class of drugs if they were able to cross cell membranes. Many chemical modifications have been developed, including thiophosphate, peptide nucleic acid, locked nucleic acid and morpholino oligonucleotides, to improve their properties. Owing to the polyanionic nature of oligonucleotides/nucleic acids, conjugation to a polycationic moiety is the rational approach to decrease charge repulsion and thus improve hybridization properties. Moreover, polycations are known to carry oligonucleotides into cells, hence their conjugates are expected to behave similarly. Various synthetic approaches for introducing cations into oligonucleotides have been described, including phosphate backbone replacement, nucleotide modifications or end-conjugation, for which enhanced hybridization, strand invasion and eventually cell penetration were observed.
Among cation-modified oligonucleotides, those leaving the oligonucleotide moiety intact retain mismatch discrimination and remain substrates of nucleic acid–processing enzymes. Moreover, molecular biology applications require rapid on-demand synthesis of oligonucleotide sequences, and this requirement was not fulfilled by previous synthetic approaches.
By conjugation of spermine derivatives as cationic moieties (Z-units) to – by nature – polyanionic oligonucleotides, a versatile concept to increase affinity of oligonucleotides for their target by decreasing electrostatic repulsions has been developed and proven.
ZNA oligos result from the conjugation of a polycationic moiety to an oligonucleotide. This decreases the charge repulsion between the oligonucleotide and its target sequence, both naturally anionic.